Multi-scale Non-equilibrium Coupled Reaction and Transport
Hu Lab Research Direction
Key Takeaway
Vision Statement

Our Work
We have utilized operando pH mapping, Raman spectroscopy, multi-physics modeling, and reactor design to leverage molecular flux catalysis in our PEC devices to convert oceanic carbon into fuels
Impact
Research Background
Our Research Question
Developing carbon capture, utilization (CCU), and storage technologies is crucial for managing anthropogenic carbon dioxide (CO2) emissions while providing sources of sustainable chemicals and fuels.
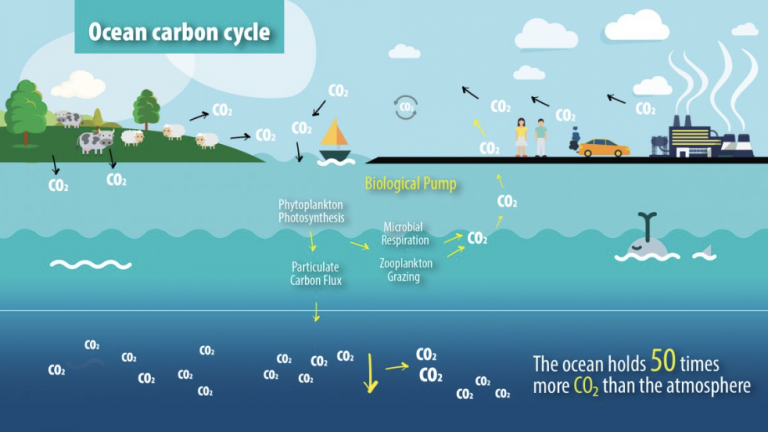
However, direct air capture is energy-intensive and costly. In comparison, dissolved inorganic carbon in seawater has a bicarbonate molarity that is ~ 140 times higher than the 420 ppm of atmospheric CO2. Furthermore, seawater is a natural carbon sink of net ~ 0.4 giga-ton CO2 per year via the flux exchange between the seawater and atmosphere, potentially supporting trillion-ton-scale CO2 capture, utilization, and sequestration via engineering solutions.
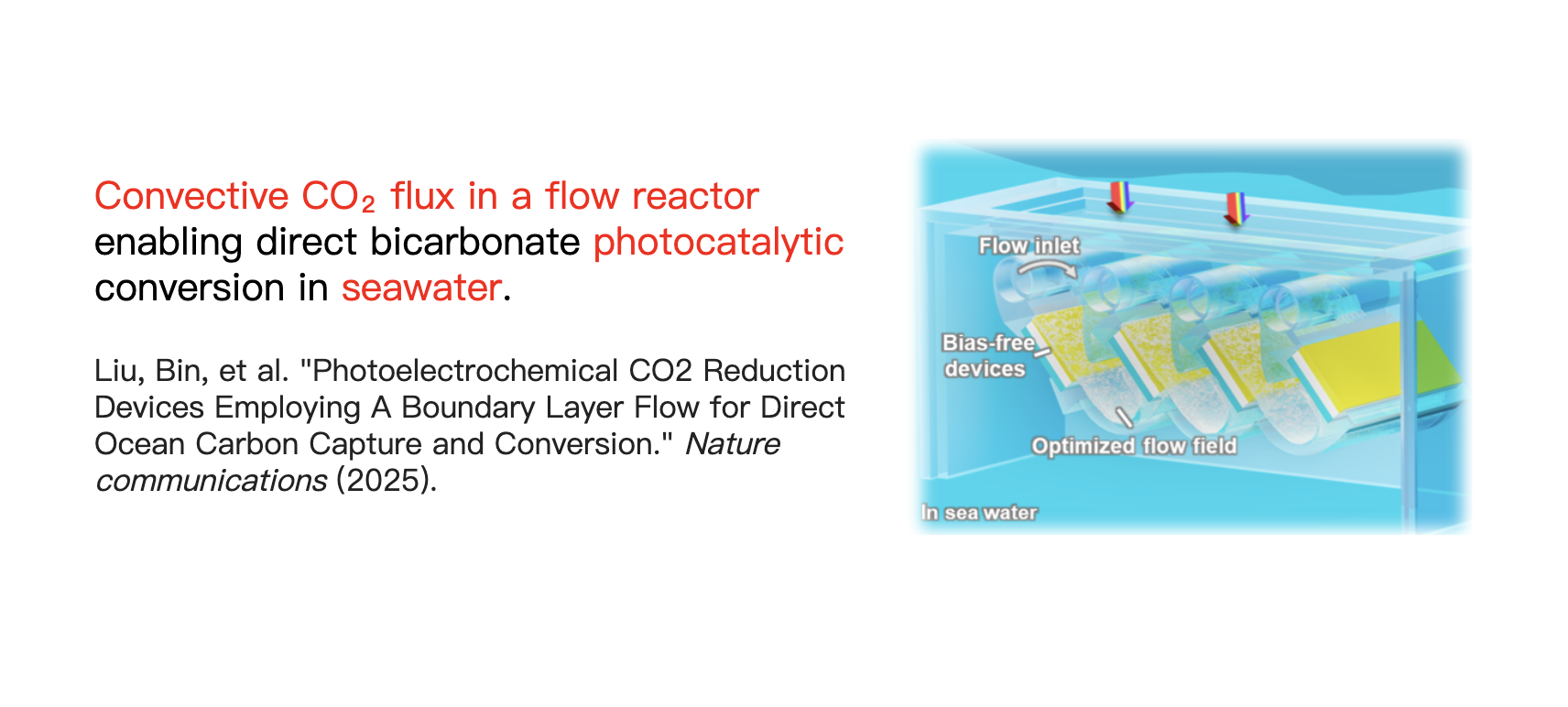
In particular, the conversion of dissolved carbon in seawater into carbon-based fuels or chemical products can be entirely powered by sunlight using photoelectrochemical (PEC) devices. Such solar-powered chemical devices, floating on the ocean, could utilize ocean current, tidal energy, and sunlight to generate dissolved CO2 on demand via bicarbonate acidification, i.e., HCO3−+H+ → CO2(aq) + H2O.
Reactive Carbon Capture and Conversion

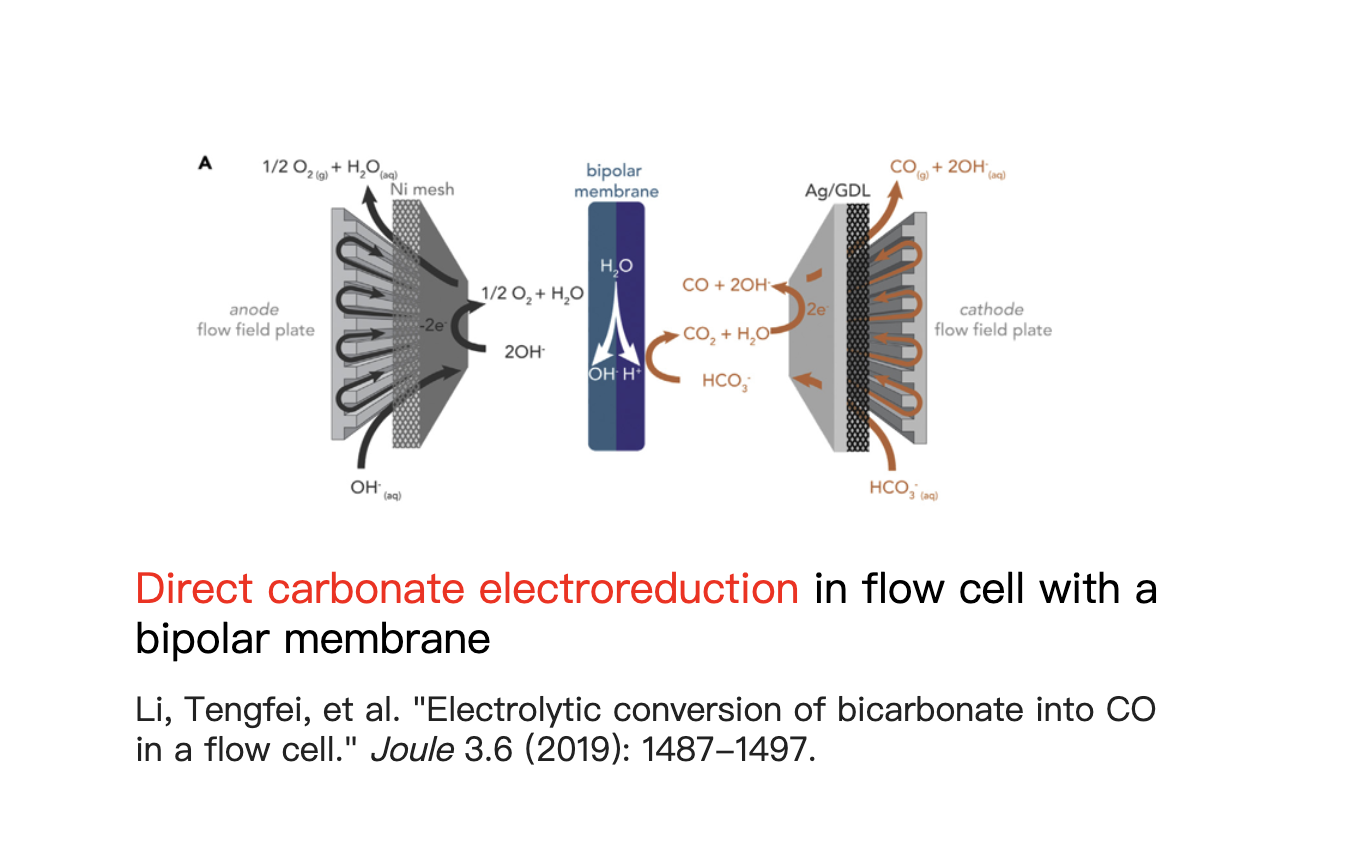

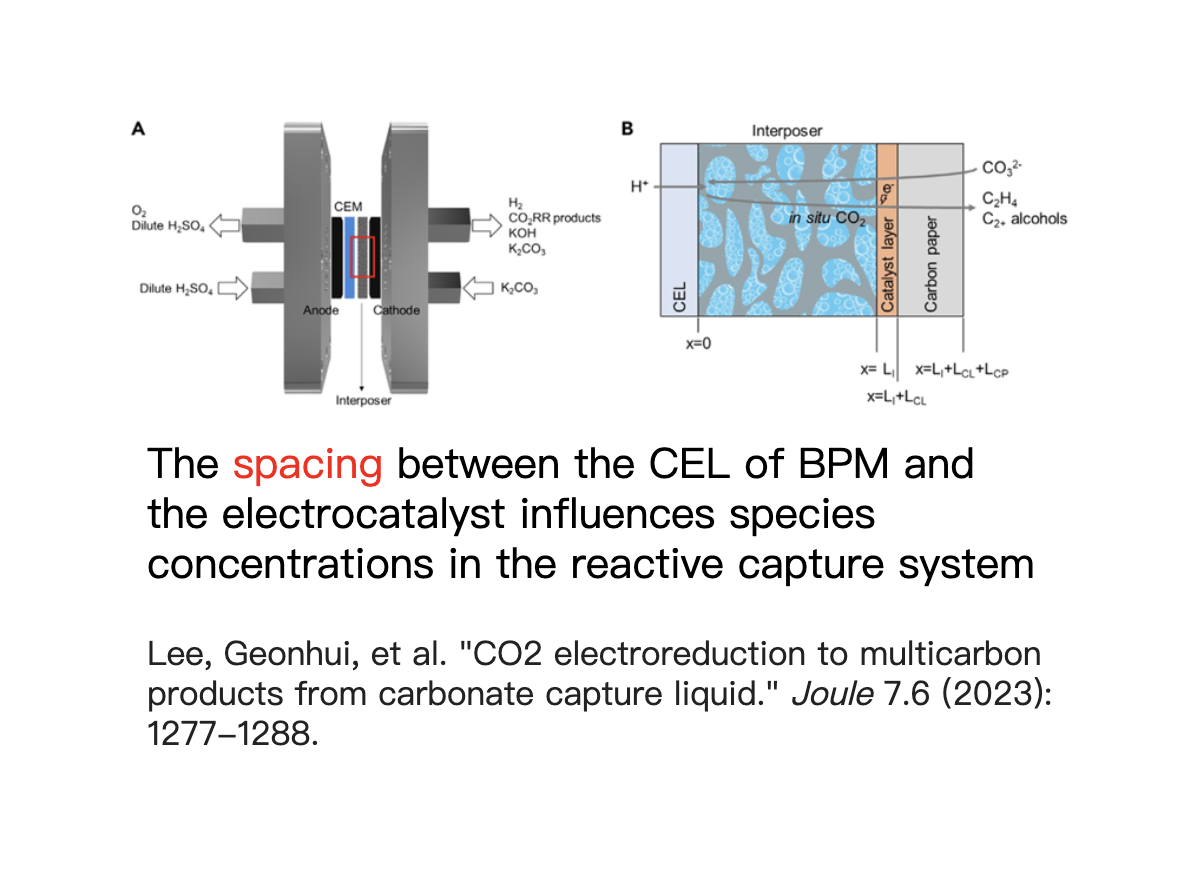
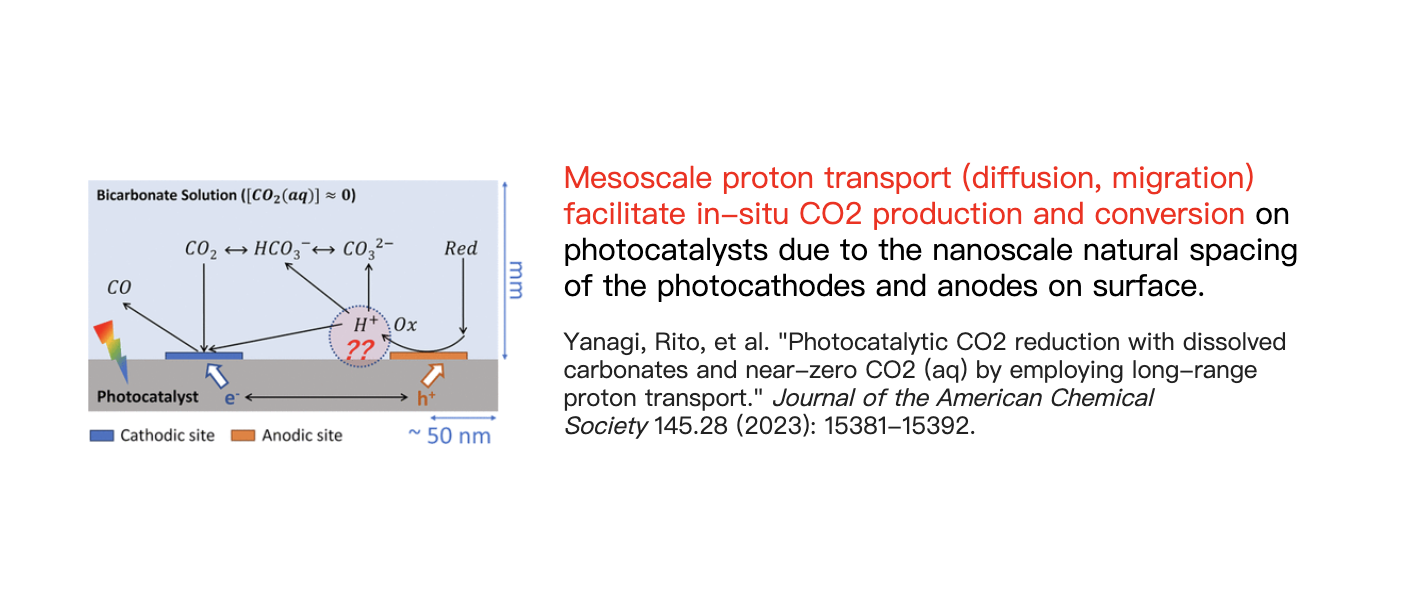
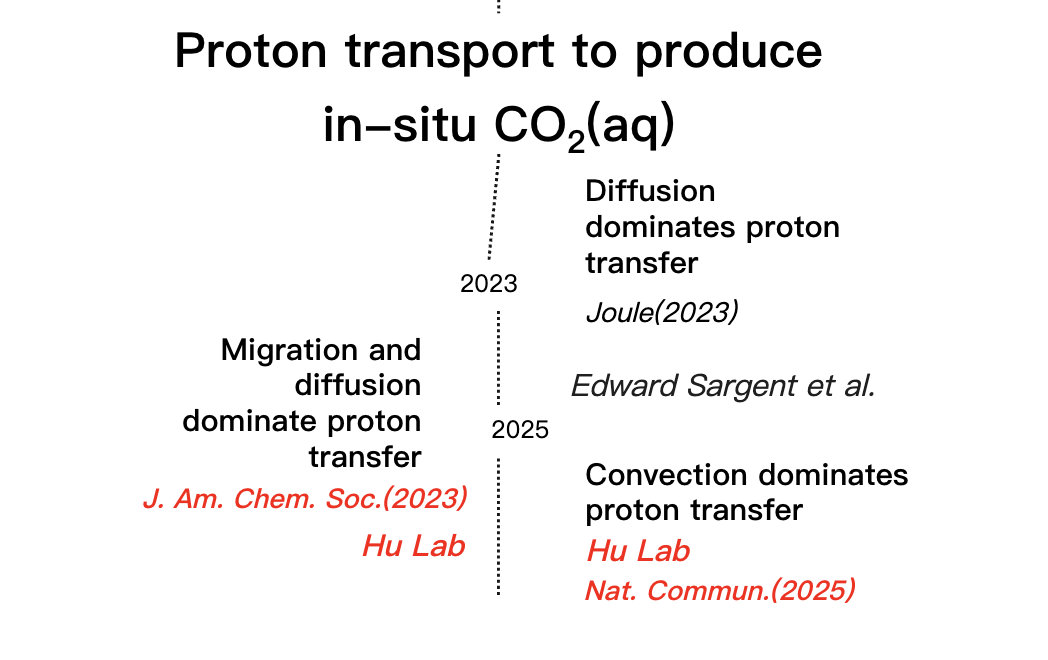
Our Research Direction
The direct utilization of dissolved inorganic carbon in seawater for CO2 conversion promises chemical production on-demand and with zero carbon footprint. PEC CO2RR devices promise the sustainable conversion of dissolved carbon in seawater to carbon products using sunlight as the only energy input.
However, the diffusion-dominant transport mechanism and the near-zero concentration of CO2(aq) (CO2 dissolved in aqueous solution) in static seawater has made it extremely challenging to achieve high solar-to-fuel (STF) efficiency and high carbon–product selectivity. The catalytic reaction rate, which is a primary interest of CO2RR devices, is jointly determined by the kinetic rates of the catalyst and the mass transport efficiency.
Here, we discuss the concept of “Molecular flux”, using examples of how high CO2 flux can be generated from the anode to the cathode through electric-field-coupled or flow-coupled reactive transport in seawater.
When the reaction is dominated by reactant mass transport − particularly when the reactant concentration in the bulk solution approaches 0 millimolar, as in the case of dissolved CO2 − improving the overall efficiency requires generating a net reactant molecular flux. Molecular flux catalysis refers to a scenario in which a cascade reaction occurring at one electrode generates the reactant flux for the counter electrode, thereby synergistically facilitating molecular conversion.
Our Research
We co-developed innovative photocatalysts and flow reactors to address the challenge of low-cost, efficient reactant utilization in practical solar photosynthesis applications. Our research focuses on optimizing these systems to enhance performance under real-world conditions.
Molecular Flux via E-field
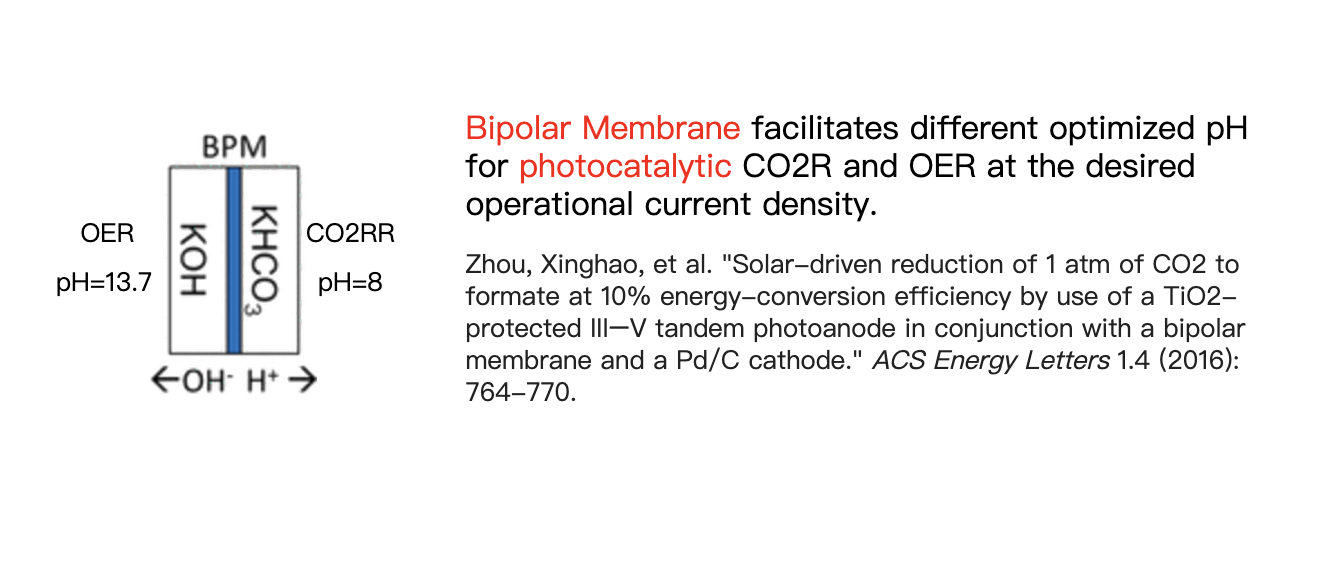
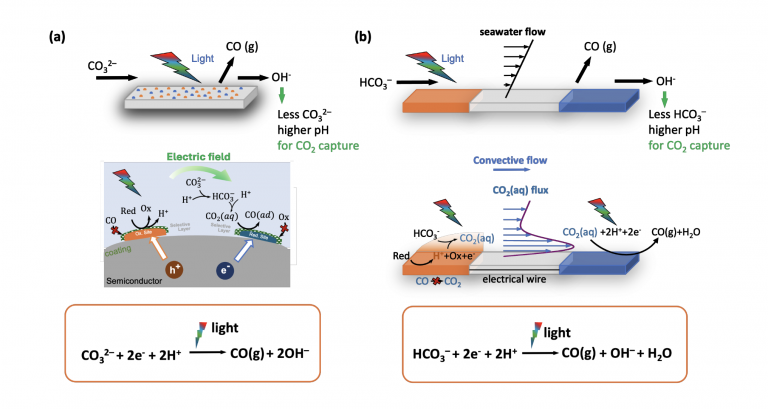
Specifically, as shown in Figure a, after the oxidation reaction at the anode, which produces protons, protons rapidly migrate to the nearby cathode under an electric field. During this process, protons combine with bicarbonate ions in the solution to form reactive CO2(aq) molecular flux, which is then reduced at the cathode. The total molecular flux from anode to cathode plays a pivotal role in the overall reaction.
In this context, we discovered a novel approach to studying catalysis and adsorption under non-equilibrium dissolved CO2(aq) flux conditions. Unlike traditional CO2 reduction processes, which operate under equilibrium conditions, we achieved significant CO2 adsorption even when the local CO2(aq) concentration at pH 12.5 was nearly zero.
This breakthrough was facilitated by the use of Ag-CrOₓ co-catalysts to modify coated GaInP, resulting in 100% CO selectivity under CO2(aq) flux conditions (JACS 2023).
Molecular Flux via Convection
Due to the nature of charge separation on photocatalysts, the distance between the cathodes and anodes is limited to a few hundred nanometers. At this scale, the electric field between the electrodes significantly affects the mass transfer process.
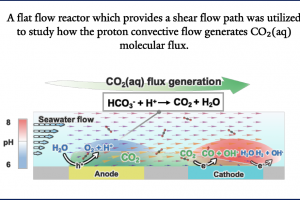
Following a similar philosophy, convection can also bring the proton generated at the anode to the downstream cathode where the CO2RR takes place. Compared to the change in flux caused by the variation in laminar flow velocity with distance from the bottom wall, the CO2 flux exhibits a sharp increase near the bottom surface. This increase is attributed to the protons generated by the oxygen evolution reaction at the upstream cathode, which combine with bicarbonate ions in the solution under flow to produce CO2 (as indicated by the purple line in Figure b).
Solar-driven Selective Conversion of Millimolar Dissolved Carbon to Fuels with Molecular Flux Generation
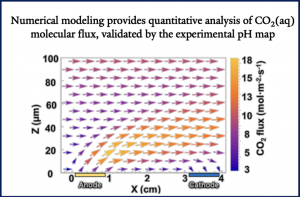
The ocean, as the world’s largest dynamic CO₂ reservoir, has a dissolved inorganic carbon concentration 140 times that of CO₂ in the atmosphere. If the conversion of bicarbonate in seawater can be achieved, it would eliminate the need for the CO₂ capture and enrichment process prior to reducing gaseous products, significantly lowering costs and providing a feasible direction for industrial scale-up. However, the concentration of reactive CO₂(aq) in seawater is virtually zero, making it challenging for traditional photoelectrochemical devices to achieve efficient CO₂ reduction reactions.
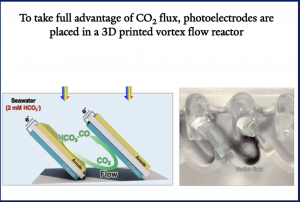
To utilize dissolved carbon in seawater, we recently innovated by utilizing proton convective flows instead of proton migration flux to generate CO₂(aq) molecular flux. This novel design employs shear flows to confine CO₂(aq) flux near the surfaces of CO₂R photocathodes.
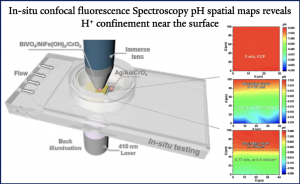
Additionally, the spatial pH distribution was monitored in situ using confocal fluorescence spectroscopy. Under static conditions, the pH near the BiVO₄ photoanode surface (0-100 μm) decreased to approximately 4.3. As the flow rate increased, the pH gradient was confined to a thinner boundary layer. This flow not only restricted proton diffusion but also holds significant implications for enhancing CO₂ extraction and conversion efficiency.
Through COMSOL multiphysics simulations, the effects of flow on mass transport and reaction selectivity were investigated. The pH data obtained from the simulations align with the experimental pH map, demonstrating the reliability of the simulations. Simulation results indicate that at a flow velocity of 0.77 m/s, the CO₂ flux can reach 18.8 mmol·m⁻²·s⁻¹.
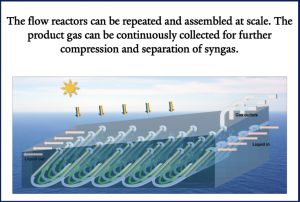
By integrating computational fluid dynamics and 3D printing, we designed a vortex system where H+-producing photoanodes are positioned upstream of a CO2RR photocathode downstream to maximize the utilization of the molecular flux generated at the anodes. This configuration leverages natural ocean currents, allowing the solar fuel device to operate efficiently while floating
These advancements enabled us to achieve a record-high 51% CO selectivity for syngas production from 2 millimolar dissolved bicarbonate in seawater.
The study introduces a novel concept of molecular laminar flow catalysis, addressing the issue of low carbon dioxide concentration in seawater and enabling efficient and directional photo conversion of carbon in seawater. A well-defined boundary layer flow between the BiVO₄ photoanode and the Si photocathode within a photoelectrochemical device was established, enhancing the CO selectivity from 3% to 21%, while achieving a solar-to-fuel conversion efficiency of 0.71%.
Future Work
From now on, the redox reaction near active sites needs to be quantified, together with the active sites themselves.
Due to the reaction kinetics in the carbonate system, the non-equilibrium reactive flux exhibits both high pH and relatively high CO2 concentration, a regime in which adsorption and reaction selectivity remain unexplored.
Currently, we are probing water-splitting semiconductor-liquid interfaces via a digital-twin/physical-twin approach: this method makes it possible to use a single multi-physics digital model to process the data from multiple individual and dissimilar characterization approaches successfully, owing to the rapid development of AI/ML (physics-informed neural network ). In a sense, self-driving cars use a similar system but only based on classic mechanics. The Hu lab is trying to obtain full information on electrochemical interfaces during dynamic operation. This digital-twin approach can combine dissimilar yet relevant approaches of electron microscopy, optical fluorescent/reflection microscopy, and nanoscale electrochemical microscopy.
Our AL/ML/DT approach makes it possible to elucidate the dynamic chemical processes across multiple scales. It paves the way for discovering easily manufacturable photocatalysts that operate the most efficiently in scale-up reactors. One immediate impact is to design and demonstrate photocatalysts operating at a record rate for ocean-based CCU.
Conclusion
Our understanding on molecular flux enables reactive carbon capture and utilization (CCU) in one step, where a continuous supply of (bi-)carbonate solutions can replace the bubbling of pure CO2 gas as a conventional source for CO2 conversion.
We achieved molecular flux driven by proton migration through the electric field between the photocatalyst cathodes and anodes, enabling efficient operation in a carbonate solution where the concentration of CO₂(aq) is essentially zero.
We also established flow-based flux catalysis, where direct photoelectrochemical conversion of bicarbonate in seawater selectively to CO gas, without gaseous CO2 bubbling, was achieved by introducing and tuning the convective transport flux from water-oxidation photoanodes and CO2-reduction cathodes of cascade reaction pairs.
Specifically, under ~ 0 mM CO2 concentration, a CO2(aq) molecular flux of ~20 mol m-2 s-1 achieved 0.5 mA cm-2 carbon-monoxide-production partial currents, equivalent to that with 2 mM CO2(aq). A record 0.71% solar-to-fuel conversion efficiency was achieved in seawater.
This mode of flux catalysis is being applied to various redox reactions and even radical-controlled reactions, including water splitting, hydrogen peroxide production, and methane selective oxidation.
Different from traditional electro- and thermal-catalysis, the elementary reaction steps, such as proton reduction and proton-coupled water oxidation, are spatially separated and varying.
Furthermore, navigating scale-up from a laboratory-scale reactor to an industrial-scale reactor capable of long-term operation while maintaining high efficiency and stability will also be a key direction for future development.
Acknowledgements & References
References:
Bin Liu, et al. “Photoelectrochemical Conversion of Dissolved Carbon in Seawater to Fuels under CO2(aq) Molecular Flux”, Nature Communications, 16, 1558 (2025). DOI: 10.1038/s41467-025-56106-3
Yanagi, Rito, et al., “Photocatalytic CO2 Reduction with Dissolved Carbonates and Near-Zero CO2(aq) by Employing Long-Range Proton Transport”, Journal of the American Chemical Society, 145, 28, 15381-15392 (2023). DOI: 10.1021/jacs.3c03281